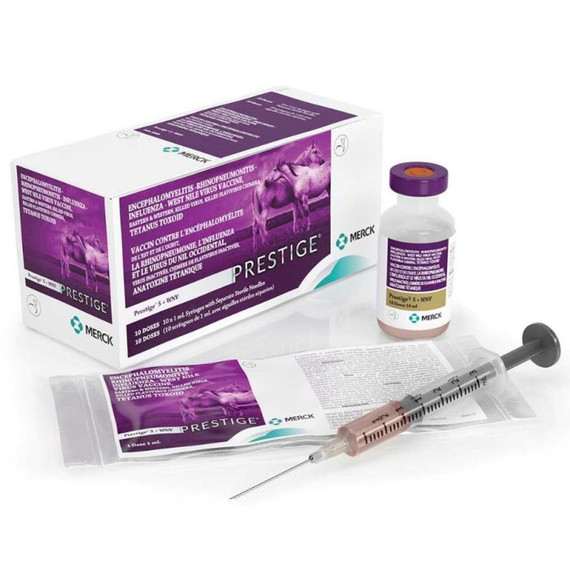
Merck Prestige V With Havlogen Equine Vaccine - 1 ml
For vaccination of healthy horses 6 M of age or older, as an aid in the prevention of disease caused by eastern and western encephalomyelitis viruses and tetanus, as an aid in the control of respiratory disease caused by EIV, EHV-1 And EHV-4 And as an aid in the reduction of virus shedding of EIV And EHV-1. Duration Of Immunity (DOI) Has been shown to be at least 6 M for EIV.
Advantages
- Multiple antigen formulation reduces the need for several injections
- Formulated to minimize reactions and maximize protection
- Available in 10-dose vials. Also available in convenient, low-volume 1 ml doses in single, prefilled syringes
- Tetanus, EEE And WEE ARe core vaccinations recommended by the AAEP Vaccine guidelines and are indicated in the immunization program for all horses each year
- Provides 6 M Of Protection Against EIV
- A combination of inactivated, concentrated, adjuvanted, tissue culture origin equine encephalomyelitis viruses (eastern and western), equine herpesviruses types 1 and 4 (EHV-1 And EHV-4), Equine Influenza Virus Type H3N8 Strains (EIV A/Equine 2/ Kentucky/93 American, EIV A/Equine 2/Kentucky/02 American And EIV A/Equine 2/Newmarket/2/93 Eurasian) and Tetanus Toxoid. Intervet Serological Data Suggest Influenza Cross-protection Against Certain U.S. and European Strains, Including Kentucky 91, 93, 99, 02, Ohio 03, Newmarket/1/93, Newmarket/2/93, Suffolk 89 and South Africa 03
- Exceptional safety profile with the smooth havlogen adjuvant
- Advanced filtration process through the antigen purification system (APS)
Administration And Dosage
For primary vaccination aseptically administer 1 ml intramuscularly, repeat with a single dose in three to four weeks. Annual revaccination is recommended. A booster dose can be administered at more frequent intervals based upon individual horse or farm disease risk assessment or any time epidemic conditions exist or are reported. Consult your veterinarian.
Precautions
Store At 2°-7°C (35°-45°F). Shake well before using. Use entire contents when first opened. Do not vaccinate within 21 days before slaughter. Inject deep into the muscle only. Local reactions may occur at the injection sites in vaccinates. Systemic reactions may also occur in animals following vaccination. If an allergic reaction occurs, treat with epinephrine. Care should be used to avoid injecting your own fingers, hands, or other body parts. Accidental injection can cause serious local reactions. Contact a physician immediately if an accidental injection occurs.
Specifications
- Size: 1 ml
For vaccination of healthy horses 6 M of age or older, as an aid in the prevention of disease caused by eastern and western encephalomyelitis viruses and tetanus, as an aid in the control of respiratory disease caused by EIV, EHV-1 And EHV-4 And as an aid in the reduction of virus shedding of EIV And EHV-1. Duration Of Immunity (DOI) Has been shown to be at least 6 M for EIV.
Advantages
- Multiple antigen formulation reduces the need for several injections
- Formulated to minimize reactions and maximize protection
- Available in 10-dose vials. Also available in convenient, low-volume 1 ml doses in single, prefilled syringes
- Tetanus, EEE And WEE ARe core vaccinations recommended by the AAEP Vaccine guidelines and are indicated in the immunization program for all horses each year
- Provides 6 M Of Protection Against EIV
- A combination of inactivated, concentrated, adjuvanted, tissue culture origin equine encephalomyelitis viruses (eastern and western), equine herpesviruses types 1 and 4 (EHV-1 And EHV-4), Equine Influenza Virus Type H3N8 Strains (EIV A/Equine 2/ Kentucky/93 American, EIV A/Equine 2/Kentucky/02 American And EIV A/Equine 2/Newmarket/2/93 Eurasian) and Tetanus Toxoid. Intervet Serological Data Suggest Influenza Cross-protection Against Certain U.S. and European Strains, Including Kentucky 91, 93, 99, 02, Ohio 03, Newmarket/1/93, Newmarket/2/93, Suffolk 89 and South Africa 03
- Exceptional safety profile with the smooth havlogen adjuvant
- Advanced filtration process through the antigen purification system (APS)
Administration And Dosage
For primary vaccination aseptically administer 1 ml intramuscularly, repeat with a single dose in three to four weeks. Annual revaccination is recommended. A booster dose can be administered at more frequent intervals based upon individual horse or farm disease risk assessment or any time epidemic conditions exist or are reported. Consult your veterinarian.
Precautions
Store At 2°-7°C (35°-45°F). Shake well before using. Use entire contents when first opened. Do not vaccinate within 21 days before slaughter. Inject deep into the muscle only. Local reactions may occur at the injection sites in vaccinates. Systemic reactions may also occur in animals following vaccination. If an allergic reaction occurs, treat with epinephrine. Care should be used to avoid injecting your own fingers, hands, or other body parts. Accidental injection can cause serious local reactions. Contact a physician immediately if an accidental injection occurs.
Specifications
- Size: 1 ml